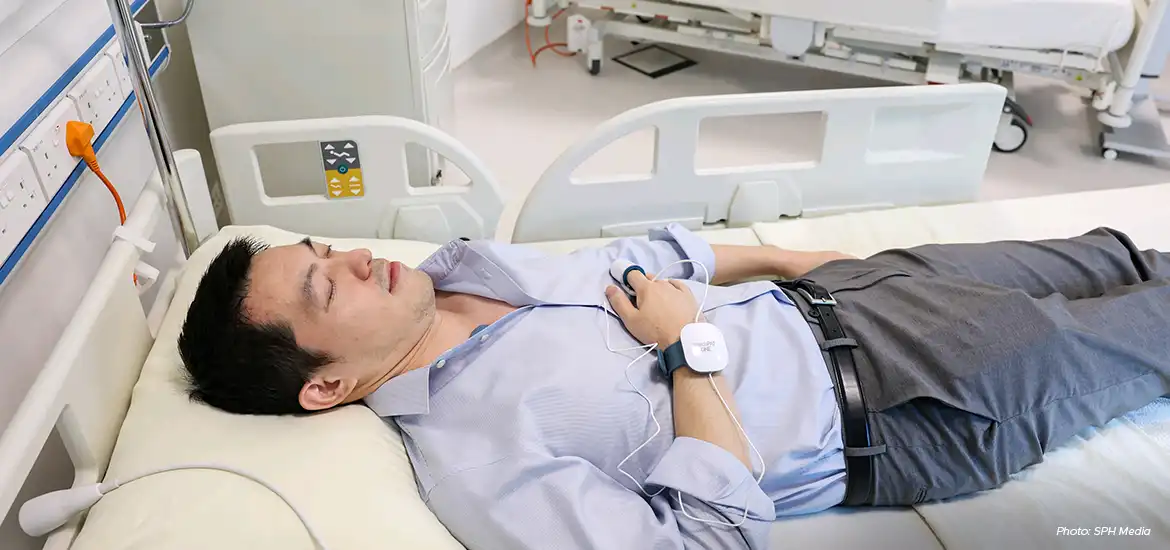
How can clinical trial participants be prevented from recording information inaccurately? One solution for pharmaceutical giant Eli Lilly and Company comes in the form of a smart toilet that detects when – and for how long – a person is using it, saving him the trouble of logging the information himself.
The smart loo is among a plethora of smart sensors used at Lilly’s new S$42 million Digital Health Innovation Hub here to make clinical trials more comfortable for participants and improve the quality of medical research.
Backed by the Economic Development Board (EDB), the hub was launched on 14 November at the Lilly Centre for Clinical Pharmacology in Buona Vista, expanding the US-based company’s work in Singapore, where it has operated for more than two decades.
The loo has pressure sensors to log how long a test participant is seated – one of many forms of data collected that helps researchers understand the effects of the medication they are testing.
Dr Jian Yang, the company’s vice-president of digital health, said the prototype is a building block to develop devices that can detect users’ toilet usage passively, possibly in the form of a smartwatch or wearable.